QUESTION
The data given are the results (reported by Hadinegoro et al. NEJM 2015) of two randomized control trials (named CYD14 and CYD15) that measured the effectiveness of an experimental dengue vaccine against a placebo vaccine for preventing hospitalization (up to 2 years after vaccination) in children because of dengue virus infection.
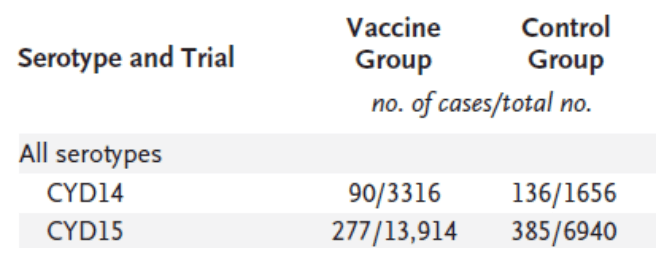
- What are the PICO elements of the research question?
- What is the measurement scale of the Outcome variable?
- Given your answer to (b) what statistic can be used to report the burden of
this outcome variable? - Given the study design used, was the outcome event that the vaccinated
children were being followed-up for a “good” or “bad” event? - What was the period of follow-up for this Outcome event?
- If the vaccine is truly MORE effective than a placebo, would you expect the
chance of this outcome event to be higher or lower in the vaccine group
compared to the control group, give your reason - Create and properly label two contingency tables (one for each study) to
calculate the vaccine’s treatment effect (show working). You should
calculate and report the treatment effect in the following 3 ways – risk
ratio (RR), risk difference (RD), and odds ratio (OR). - From the treatment effects that you have just calculated in the CYD14
study, is there evidence that the experimental vaccine is more effective
than the placebo vaccine? Using the risk difference statistic, describe how
much more or less effective the experimental vaccine is. - Using the odds ratio statistic from the CYD15 study, describe to a lay
person how much more or less effective the experimental vaccine is.
